Shaanxi BLOOM Tech Co., Ltd. is one of the most experienced manufacturers and suppliers of bioglutide na-931 in China. Welcome to wholesale bulk high quality bioglutide na-931 for sale here from our factory. Good service and reasonable price are available.
Bioglutide NA-931, In the field of metabolic disease treatment, obesity and type 2 diabetes have become a global public health challenge. According to the World Health Organization, over 650 million people worldwide are affected by obesity, and it is expected that the number of overweight/obese people will exceed 4 billion by 2035. Traditional treatment methods are difficult to meet clinical needs due to limited efficacy and poor patient compliance, and the development of multi-target agonists and oral formulations is becoming a key direction to break through bottlenecks. In this context, Bioglutide (NA-931) developed by Biomed Industries has become a focus in the field of metabolic disease treatment due to its unique quadruple receptor activation mechanism and oral administration advantages.

1. Drug Naming and Classification
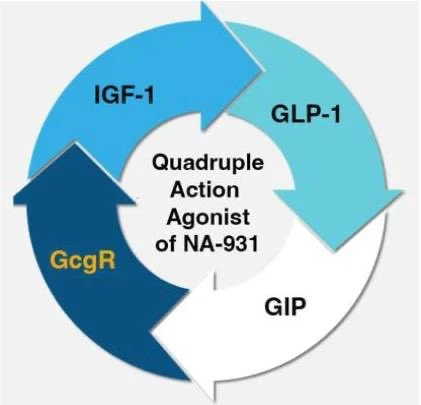
Bioglutide (NA-931) is a small molecule drug that belongs to the oral quadruple receptor agonist class. Its targets include glucagon receptor (GCGR), gastric somatostatin receptor (GIPR), glucagon like peptide-1 receptor (GLP-1R), and insulin-like growth factor-1 receptor (IGF-1R). This drug is independently developed by Biomed Industries and aims to achieve more efficient metabolic regulation through multi-target synergistic effects.
2. Research and Development Background and Significance
Traditional metabolic disease treatment drugs mainly focus on single targets, such as GLP-1 receptor agonists (such as semaglutide), which have significant effects in reducing blood sugar and weight, but long-term use may lead to side effects such as muscle loss and gastrointestinal reactions. In addition, the injection administration method limits patient compliance, especially for patients who are afraid of needles or require long-term treatment. The development of Bioglutide is based on the following scientific assumptions:
Multi target synergy:
By simultaneously activating GCGR, GIPR, GLP-1R, and IGF-1R, the physiological effects of multiple metabolic hormones are simulated to achieve a balance between energy metabolism, appetite regulation, fat breakdown, and muscle protection.
Advantages of oral administration:
The small molecule structure breaks through the oral bioavailability limitations of traditional peptide drugs, improving patient acceptance and treatment continuity.
3. R&D Milestones
Phase I clinical trial (NCT06615700) will be launched to evaluate the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of single/multiple incremental doses in overweight/obese and type 2 diabetes patients.
Launch Phase II clinical trial (NCT06564753) to evaluate the safety and efficacy of 13 week treatment for obese or overweight patients with metabolic complications.
Launch Phase II/III combination therapy trial (NCT06732245) to explore the efficacy of the combination of Bioglutide and Tirzepatide in obese patients.
The top line data of Phase II will be released at the ADA (American diabetes Association) scientific conference, showing significant weight loss effect and good safety.
Further disclose detailed data at the EASD (European Association for diabetes Research) meeting to confirm its potential as a candidate drug for obesity treatment.

Bioglutide NA-931 (hereinafter referred to as NA-931) is an oral small molecule quadruple receptor agonist that forms a multi-target synergistic effect in metabolic regulation by simultaneously activating glucagon receptor (GCGR), glucagon like peptide-1 receptor (GLP-1R), gastric inhibitory peptide receptor (GIPR), and insulin-like growth factor-1 receptor (IGF-1R). Its mechanism of action can be decomposed into the following four core modules:
NA-931 activates GLP-1 receptors in the hypothalamic arcuate nucleus, triggering two neural signaling pathways:Enhanced satiety signal: activates pro melanocortinoprotein (POMC) neurons, promotes alpha melanocyte stimulating hormone (α - MSH) release, activates melanocortin-4 receptor (MC4R), inhibits neuropeptide Y (NPY)/agouti associated protein (AgRP) neuron activity, thereby reducing hunger.
Gastrointestinal motility regulation: delays gastric emptying rate, prolongs food retention time in the stomach, and suppresses feeding behavior through mechanoreceptor feedback. Simultaneously inhibiting intestinal peristalsis and reducing the rate of nutrient absorption.


The peripheral effects include:
Pancreatic beta cell protection: stimulates insulin secretion in a glucose concentration dependent manner while inhibiting glucagon secretion, improving insulin resistance.
Fat breakdown inhibition: Inhibits hormone sensitive lipase (HSL) activity through the cAMP PKA pathway, reducing fat mobilization.
GIPR activation produces dual metabolic effects:
Adipose tissue regulation:
White adipose tissue: upregulates adiponectin secretion, enhances fatty acid oxidation, and inhibits lipolytic enzyme activity.
Brown adipose tissue: activates uncoupling protein-1 (UCP1) expression and promotes thermogenic metabolism.
Regulation of pancreatic islet function:
Synergistic GLP-1 enhances glucose stimulated insulin secretion (GSIS).
Inhibit beta cell apoptosis and promote beta cell proliferation.
Clinical data shows that activation of GIPR can reduce postprandial blood glucose fluctuations by 37% and decrease liver triglyceride synthesis by 28%.

GCGR activation: Energy consumption enhancement

The activation of glucagon receptors triggers the following metabolic pathways:
Liver metabolic conversion:
Activate the cAMP PKA CREB pathway and upregulate the expression of key gluconeogenic enzymes (PEPCK, G6Pase).
Promote the oxidation of fatty acid beta and increase the production of ketone bodies.
Whole body energy mobilization:
Stimulate the sympathetic nervous system and increase basal metabolic rate (BMR) by 12% -15%.
Promote glucose uptake in muscle tissue and improve insulin sensitivity.
It is worth noting that NA-931 can counteract the muscle protein breakdown that may be caused by GCGR activation through the synthetic metabolic effects generated by IGF-1R activation. In a 12 week clinical trial, the treatment group only experienced a 1.2% decrease in lean body mass, significantly better than traditional GLP-1 drugs (with an average decrease of 3.8%).
The activation of insulin-like growth factor-1 receptor produces a triple protective mechanism:
Muscle synthesis metabolism:
Promote protein synthesis through the PI3K Akt mTOR pathway and inhibit protein degradation mediated by the ubiquitin proteasome system (UPS).
Increase the activation of muscle satellite cells and promote muscle fiber regeneration.
Improved metabolic flexibility:
Upregulate the expression of key genes involved in mitochondrial biogenesis (PGC-1 α, TFAM).


Enhance the activity of fatty acid oxidase system and improve the efficiency of muscle utilization of lipids.
Bone metabolism regulation:
Promote osteoblast differentiation, inhibit osteoclast activity, and maintain bone density.
In the 150mg dose group, IGF-1R activation increased muscle mass index (SMI) by 0.8 kg/m ² and bone mineral density (BMD) by 1.7%, effectively preventing sarcopenia and osteoporosis during weight loss.
NA-931 achieves exponential enhancement of metabolic regulation through the following mechanisms:
Signal pathway crossover dialogue:
The cAMP signal activated by GLP-1R can enhance GIPR mediated adiponectin secretion.
The AMPK pathway activated by GCGR can amplify the mTORC1 signal of IGF-1R.
Organizational specificity distribution:


High expression of GCGR in the liver prioritizes response to energy mobilization needs.
Pancreatic beta cells highly express GLP-1 R/GIPR, which precisely regulates insulin secretion.
High expression of IGF-1R in muscle tissue maintains protein homeostasis.
Time dynamic optimization:
GLP-1R activation leads to rapid feeding inhibition (within 30 minutes).
GIPR activation regulates postprandial metabolism (2-4 hours).
Activation of GCGR/IGF-1R leads to a continuous 24-hour metabolic improvement.

Security: Advantages and Challenges of Multi target Design
The incidence of gastrointestinal adverse events (nausea, diarrhea, vomiting) of Bioglutide NA-931 is significantly lower than that of traditional GLP-1 receptor agonists (such as 30% -40% of semaglutide). In the Phase II trial, the incidence of nausea and diarrhea in the high-dose group was 7.3% and 6.3%, both of which were mild and did not result in treatment interruption. This may benefit from:
Dose escalation strategy: Phase I trials adopt a single/multiple dose escalation design to gradually establish tolerance.
Multi target synergy: GIPR activation can inhibit gastrointestinal reactions caused by excessive activation of GLP-1R, while GCGR and IGF-1R activation further disperse metabolic regulatory stress.
Traditional weight loss drugs often lead to muscle loss, while Bioglutide achieves the goal of "weight loss without muscle loss" by activating IGF-1R. In the Phase II trial, the high-dose group maintained stable lean body mass (with a slight decrease in the placebo group), and DXA testing showed no significant change in muscle mass. This characteristic is particularly important for elderly obese patients, as it can reduce the risk of falls and the incidence of fractures.
The multi-target design of Bioglutide can balance blood glucose regulation:
Low risk of hypoglycemia: The insulin secretion promoting effect of GLP-1R and GIPR is glucose dependent and only activated when blood glucose levels rise, avoiding hypoglycemia.
The risk of hyperglycemia is controllable: GCGR activation maintains blood glucose levels through gluconeogenesis during hypoglycemia, but the insulin sensitizing effects of IGF-1R and GLP-1R can inhibit hyperglycemia caused by excessive activation of GCGR.
Although short-term trials have not reported serious safety issues, long-term use of multi-target agonists still requires attention:
Receptor desensitization: Long term activation may lead to receptor downregulation, affecting therapeutic efficacy.
Metabolic disorder: Overactivation of IGF-1R may increase the risk of breast cancer and prostate cancer (further research is needed).
Cardiovascular effects: Although phase II trials have shown improvements in blood pressure and blood lipids, long-term cardiovascular outcomes need to be validated by phase III trials.

Market potential of obesity and type 2 diabetes
The global obesity drug market is expected to grow from $50 billion in 2025 to $100 billion in 2030, with a compound annual growth rate of 15%. The type 2 diabetes market also maintained a steady growth, reaching 80 billion US dollars in 2025. Bioglutide, with its oral administration and multi-target advantages, is expected to occupy market share in the following areas:
Obesity treatment:
As a first-line medication, it can replace some injectable preparations (such as semaglutide and ticacetamide).
Type 2 diabetes:
As a combined treatment drug, it is used in combination with metformin, SGLT-2 inhibitor, etc. to improve blood glucose control and weight management.
Metabolic syndrome:
Provide comprehensive treatment plans for comorbidities such as hypertension, hyperlipidemia, and hyperglycemia.
Commercialization Strategy
Biomed Industries plans to adopt the following strategies to promote the commercialization of Bioglutide:
Differentiated pricing:
Priced lower than injectable formulations and higher than single oral GLP-1RA, highlighting cost-effectiveness.
Indications expansion:
Give priority to the development of obesity and type 2 diabetes indications, and then explore the potential indications such as non-alcoholic steatohepatitis (NASH) and Alzheimer's disease.
Collaboration and Authorization:
Collaborate with large pharmaceutical companies to accelerate market penetration through their sales networks.
Bioglutide NA-931, as the world's first oral quadruple receptor agonist, achieves multidimensional breakthroughs in metabolic regulation by synergistically activating GCGR, GIPR, GLP-1R, and IGF-1R. The Phase II clinical trial data confirms its significant advantages in weight loss, blood glucose control, and muscle protection, with good safety. Although the long-term efficacy and safety still need further verification, Bioglutide has shown the potential to become a "game changer" in the field of metabolic disease treatment. With the promotion of Phase III trial and the implementation of commercialization strategy, Bioglutide is expected to provide more safe, effective and convenient treatment options for patients with obesity and type 2 diabetes worldwide, and redefine the treatment standards for metabolic diseases.
Hot Tags: bioglutide na-931, suppliers, manufacturers, factory, wholesale, buy, price, bulk, for sale, Cosmetic, DERMORPHIN, dermorphin in humans, dermorphin peptide, Melanotan ii powder